
William Gallentine, DO, explains the initial signs of pediatric seizures and epilepsy and how pediatricians can point them out, all while reassuring parents and determining proper care.

William Gallentine, DO, explains the initial signs of pediatric seizures and epilepsy and how pediatricians can point them out, all while reassuring parents and determining proper care.

A new systematic review and meta-analysis found that the risk of type 2 diabetes decreased significantly at walking speeds of 4 km/h or above.

Birch triterpenes (FILSUVEZ; Chiesi Global Rare Diseases) is the first approved treatment for wounds associated with junctional epidermolysis bullosa (JEB), according to the developer.

The study evaluated whether fetuses with Down syndrome and normal obstetric ultrasounds (OB-USs) had missed diagnoses of critical congenital heart disease (CHD) by conducting a retrospective review.

In a recent phase 3 trial, omalizumab (Xolair; Genentech) led to children and adolescents being able to receive higher doses of common foods without allergic reactions.
Mothers with mood disorders—either bipolar disorder, major depressive disorder— or schizophrenia/ schizoaffective disorder are not associated with their offspring’s risk for type 1 diabetes, according to a recent study.

A lead study investigator highlights epicutaneous immunotherapy using DBV 712 (Viaskin Peanut; DBV Technologies), its study details, and breaks down its usability, indications, and treatment potential.

Seborrheic dermatitis patients aged 9 years and older can now use roflumilast foam 0.3% following its approval by the FDA.
In the second installment of RSV Roundtable, our panel explains how they are educating patients and parents when it comes to RSV, vaccines and preventive measures, and limited treatment availability.

Each year in the United States, there are approximately 700 to 800 cases of NB, of which 90% are diagnosed before the age of 5 years. More than half of these cases are considered high-risk.

The impending Supreme Court decision regarding mifepristone, a crucial abortion medication, could modify access by potentially imposing restrictions and influencing the abortion rights landscape.
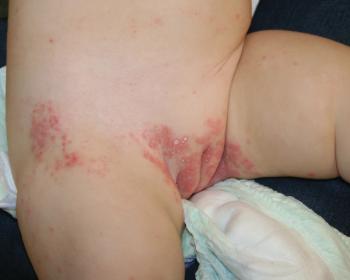
You are asked to evaluate a healthy 2-year-old girl who has developed a mildly pruritic rash with erythematous plaques, some with silvery-white scale, in the vulvovaginal area 3 months ago. What's the diagnosis?

The United States Preventive Services Task Force (USPSTF) is planning to recommend that comprehensive, intensive behavioral interventions be the primary effective intervention for weight loss in children and adolescents with high body mass indexes (BMI).
New data presented at the American Society of Hematology (ASH) Annual Meeting and Exposition revealed emicizumab-kxwh (Hemlibra; Genentech) controlled bleeding in babies up to 12 months while demonstrating safety and tolerability.

No adverse events have been reported related to the recall of Vigabatrin for Oral Solution, USP 500 mg, which is indicated as an adjunctive therapy for refractory complex partial seizures in patients aged 2 years and older.

Lawrence Eichenfield, MD, discusses topical steroid, non-steroidal, and potentially new atopic dermatitis treatments for the pediatric population.
A recently published study on 17 million children found combined flu and COVID-19 vaccines offered superior protection against hospitalization, MIS-C, and death during the pandemic.
In a study that measured blood pressure (BP) in multiple stages of life until young adulthood, investigators concluded that lower BP levels early in life, maintained through young adulthood, could reduce the risk of cardiovascular disease (CVD).

Reduced sleep efficiency and increased sleep irregularity in adolescents and young adults with type 1 diabetes are associated with higher HbA1c levels, insulin resistance, and endothelial dysfunction, highlighting the significance of addressing sleep-related factors in diabetes care for improved overall health.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

Sickle cell disease (SCD) patients aged 12 years and up can now be treated with exa-cel and lovo-cel, following simultaneous approval from the FDA on December 8, 2023.

Last year’s historic RSV season left many wondering if this was going to become a recurring trend. Physicians weigh in on what they are seeing at their institutions in terms of infection rates.

At the 2023 American Academy of Pediatrics (AAP) National Conference & Exhibition, a presenter shares how nutritional literacy can benefit pediatric health.

Tina Tan, MD, FAAP, FIDSA, FPIDS, explains that the recent uptick in pediatric pneumonia cases across the country, which some have called "white lung syndrome," is nothing new, and there is no cause for panic.

Investigators asked the children’s parents about the history of musculoskeletal-related disease, observed the children’s gait, and performed physical examinations.

To determine the association between infertility and fertility treatment with ASD risk, investigators conducted a retrospective, population-based study using health data from Ontario, Canada.

The study authors wrote that future work could prioritize data from late summer and early fall seasons to better ascertain lead time values.
Continuous glucose monitors have been found to improve sleep for parents of young children with type 1 diabetes and may help alleviate the care burden associated with the condition, according to a study conducted in France.

In this Contemporary Pediatrics interview, Lawrence Eichenfield, MD, explains additional challenges pediatric atopic dermatitis patients face during winter months and colder weather.

Investigators concluded that pharmacists at the pediatric HSSP performed clinical interventions beyond their daily clinical and operational activities, based on results from a study presented at the Health-System Pharmacists Midyear Clinical Meeting & Exhibition held in Anaheim, California, from December 3, 2023, to December 7, 2023.