Peter C. Jenkins, MD, MSc, details his study recently published in Jama Network Open, evaluating associations between pediatric emergency department readiness and mortality, and if children of all races benefited equitably.

Peter C. Jenkins, MD, MSc, details his study recently published in Jama Network Open, evaluating associations between pediatric emergency department readiness and mortality, and if children of all races benefited equitably.

James Wallace, MD, explains the various negative effects associated with school avoidance and how primary health care providers, along with parents, can catch early signs of social and separation anxiety.

The number of children who received a T1D diagnosis did not differ from children with and without SARS-CoV-2 infection.

Data from a 4-year trial of preschool-aged children offers new insight into the effects of initiating obesity management strategies among children in early life.

Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN, shares her thoughts on the latest issue of Contemporary Pediatrics, including her concern with using ChatGPT for medical information and decisions.

Ahead of the first fall and winter virus seasons in which vaccines are available for COVID-19, influenza, and respiratory syncytial virus (RSV), the Centers for Disease Control and Prevention is recommending Pfizer’s maternal vaccine to protect newborns from severe RSV illness.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

In a recent study, women with a history of dysmenorrhea were at a significantly increased risk of endometriosis.

In a recent study, increased rates of precocious puberty were observed during the COVID-19 pandemic compared to prior years.

In this Contemporary Pediatrics interview, James Wallace, MD, discusses increased trends in school avoidance and the negative effects associated with less days in the classroom.
Positive topline results from a pair of identical phase 3 trials support the submission of a supplemental New Drug Application to the FDA for Arcutis Biotherapeutics’ roflumilast cream 0.15%, a once-daily topical to treat mild to moderate atopic dermatitis (AD) in children 6 years or older.
Based on recent positive phase 3 results, Arcutis Biotherapeutics,. intends to submit a supplemental New Drug Application with the FDA for roflumilast cream 0.05% to treat mild to moderate atopic dermatitis in children aged 2 to 5 years.

The FDA issued the Complete Response Letter (CRL) based on the request for a completed pharmacokinetic/pharmacodynamic study to assess how repeat doses of the epinephrine nasal spray compare to repeat doses of an epinephrine injection product. ARS Pharma is set to file a Formal Dispute Resolution Request to appeal the CRL.

Francheska M. Merced-Nieves, PhD, Assistant professor, Departments of Pediatrics and the Institute for Exposomic Research of Environmental Medicine & Public Health, Icahn School of Medicine at Mount Sinai, explains the associations prenatal exposure to a metal mixture and the potential negative effects for the infant.

In a recent study, the 2013 WHO criteria for diagnosing gestational diabetes mellitus had a low sensitivity when used in individuals in low-risk early pregnancy.

According to a recent C.S. Mott Children’s Hospital National Poll on Children’s Health, the recent wildfires and extreme weather events that are creating poor or unhealthy air quality are also causing concern for parents in regards to their children's health.
Standout updates include a relaxed recommendation for patients with a history of egg allergies, and that all available influenza vaccines for the 2023 to 2024 flu season are quadrivalent.

This new data expands upon limited current research on environmental elements that contribute to the extensive variation in symptoms and infection frequency seen in children.

How pediatricians can best manage electronic health records without compromising patient care.

Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

The federal agency says infants of a certain age should receive this monoclonal antibody to protect against the virus.

Two infant formula products are being voluntarily recalled by the LittleOak Company after the FDA deemed they were illegally sold in the United States.
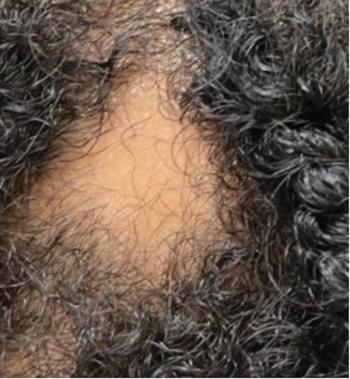
Hair loss is a condition that is often distressing and difficult to treat, particularly in adolescents. Here’s what you should know.

Surveys collected for a study published in the Canadian Journal of Diabetes revealed that desire for telehealth care amid pediatric diabetes families increased from the early onset of the COVID-19 pandemic, to more than 1 year later.

In this interview originally conducted by our sister publication Contemporary OB/GYN, Marci Bowers, MD, shares the biggest takeaways from her presentation on transgender reproductive care, presented at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

A look at the advantages and limitations of these platforms.

The 2023 guideline for managing diabetes in children and adolescents highlights risk factors and treatment for type 1 and type 2 diabetes.

In a recent study, strategies and goals for adolescent engagement with patient portals differed across US hospitals.

A supplemental biologics license application for ALTUVIIIO (Antihemophilic factor; Sanofi) has been accepted by the FDA for use in patients aged 12 years and younger with severe hemophilia A.
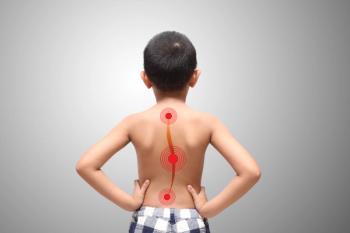
In a recent study, the prediction accuracy of an AlignProCARE model for adolescent idiopathic scoliosis was often better than that of clinicians.