
Delandistrogene moxeparvovec-rokl (ELEVIDYS) showed significant motor function improvements in 8- to 9-year-old Duchenne muscular dystrophy patients in part 2 of the phase 3 EMBARK study.

Delandistrogene moxeparvovec-rokl (ELEVIDYS) showed significant motor function improvements in 8- to 9-year-old Duchenne muscular dystrophy patients in part 2 of the phase 3 EMBARK study.

Music therapist Jenna Marcovitz discusses the role of pediatricians in making timely referrals to music therapy to support whole-child health.

Somapacitan was non-inferior and had a similar safety profile and clinical outcomes compared to once-daily somatropin in improving yearly growth rates.

The nearly 4000 cases in 2023 were the highest number reported in over 30 years, the Task Force stated.

Safety, phosphate level gains, and immunogenicity data offer encouraging signals in the pivotal trial.

The federal agency provided BioCryst Pharmaceuticals a target action date of September 12, 2025 for potential approval of berotralstat (ORLADEYO).

The administration has set a goal date of October 31 to complete a safety review, public comment period, and to take "appropriate action" in efforts to remove products from the market.

According to the agency, it is seeking public input to "determine whether existing nutrient requirements should be revised based on latest scientific data."

This FDA decision will help to improve clarity and diagnostic accuracy of echocardiograms in pediatric patients, according to GE HealthCare.

Maternal avocado consumption during pregnancy was linked to significantly lower odds of infant food allergy at 12 months.

The in vivo hematopoietic stem cell-directed therapy received rare pediatric disease and orphan drug designations earlier this year.

"Early identification of high-risk neonates may enable targeted strategies to support respiratory health," stated the study investigators.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

In this video interview, Jenna Marcovitz, MA, MT-BC, CCLS, RMT, provides insight to music therapy and how it can benefit a wide range of pediatric patients.
"These findings support Advisory Committee on Immunization Practices’ recommendations for maternal vaccination or nirsevimab to protect against severe RSV disease in infants," wrote the MMWR study investigators.
Subgroup data show crinecerfont helps children with CAH reduce steroid use while maintaining or improving androstenedione levels across diverse patient groups.

Zhang Meng shares how advancements in technology have helped prevent early cavities in childhood, and how to counsel your young patients on good oral health.

Peter S. Jensen, MD, discusses the risks and benefits of social media use by children, emphasizing the need for parental oversight and national guidelines.

NEC is a life-threatening condition marked by the death of intestinal tissue, which impacts preterm infants.

In this interview, Suzanne Hollander, MS, RD, LDN, discusses the importance of collaborative care in PKU and the impact of a new potential treatment.
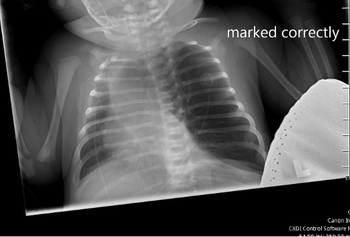
On physical examination, the patient appeared to be in significant respiratory distress. Her oxygen saturation was 96% on high-flow nasal cannula.

Prescriptions for the epinephrine nasal spray are now available for patients aged 4 years and up who weigh 33 to < 66 lbs.

A review of the clinical potential of PTC Therapeutics' sepiapterin as a novel treatment for phenylketonuria (PKU).

Even before the change in national leadership, access to adolescent gender-affirming care was becoming more difficult, with many barriers faced by these at-risk patients.
Current prevention strategies include 2 injectables for infants and young children, namely palivizumab and nirsevimab.

Research shows that nearly 80% of foster children have at least 1 significant mental health need.

Joshua Feder, MD, highlights the need for psychiatry to better integrate sensory processing research into care strategies beyond autism.
Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Watch some of our top clips from a number of discussions with experts who presented at PAS 2025.

Mona Shahriari, MD, discusses how RAD 2025 can equip providers with tools to improve atopic dermatitis outcomes.