
A new study published in JAMA Open Network suggests that early-life exposure to ambient ozone (O3) may contribute to an increased risk of asthma and wheeze in young children.

A new study published in JAMA Open Network suggests that early-life exposure to ambient ozone (O3) may contribute to an increased risk of asthma and wheeze in young children.

Read the full case presentation of a 21-month-old with a history of "skin tags" involving her gluteal cleft,noted at birth.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the April, 2025, issue of Contemporary Pediatrics, with a special focus on pediatric allergy awareness.

A newly published Swedish study supports other research that suggests screen time displaces sleep in multiple ways.

View our Q1 2025 recap of standout pediatric news from FDA regulatory updates, clinical trial results, and expert commentary.

Flovent was discontinued in January 2024 due to the manufacturer electing to produce only an authorized generic version of the medication.

Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Research suggests that sex-based differences in the kynurenine pathway may contribute to depression risk in adolescents.

Fathers who took 2 or more weeks of leave after the birth of their infant were more likely to report longer breastfeeding duration.

Aquestive Therapeutics reports positive pediatric study results for Anaphylm, supporting its FDA submission for treating severe allergic reactions.

A look back at the FDA submissions and regulatory decisions in the pediatric health care space from March 2025.

Submission is based on 3 clinical trials, including data from the ApproaCH Trial among children with achondroplasia.

Jay T. Rubinstein, MD, PhD, emphasized that children with hearing loss should receive genetic testing, as the investigational DB-OTO gene therapy demonstrated positive results.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

Fitusiran is administered subcutaneously starting once every 2 months for patients aged 12 years and older with hemophilia A or hemophilia B, with or without factor VIII or IX inhibitors.

A look ahead to 5 key regulatory decisions scheduled to take place during the second quarter of 2025, from a monoclonal antibody to topical psoriasis treatments.
Mesoblast's remestemcel-L (Ryoncil) was approved by the FDA on December 18, 2024 to treat SR-aGVHD in patients 2 months and older. Now, it is available for purchase.

Peter Lio, MD, offers thoughts on recent atopic dermatitis data reported for lebrikizumab.

Our editorial advisory board member Donna Hallas, , PPCNP-BC, CPNP, PMHS, FAANP, FAAN, highlights an article published in the March issue of Contemporary Pediatrics.

A new clinical trial has found that omalizumab (Xolair; Genetech, Novartis) is more effective than oral immunotherapy (OIT) in treating multi-food allergy in individuals with severe allergic reactions to small amounts of common food allergens.

Diazoxide choline has been granted breakthrough, fast track, and orphan drug designations in the United States.

13-year-old female with altered mental status, headache and emesis. What is the diagnosis?

In this Q+A interview, Craig McDonald, MD, discusses the interim, 90-day data from the phase 1/2 INSPIRE DUCHENNE trial, evaluating SGT-003, a gene therapy candidate for DMD.

The approval marks a vital step toward care for urinary tract infections, helping to reduce recurrence and improve patients’ quality of life.

Can you diagnose this patient? Take our poll and find out! Then check back for the full case, differential diagnosis, and correct diagnosis.

Discover mental health screening tools for the pediatric primary care provider.
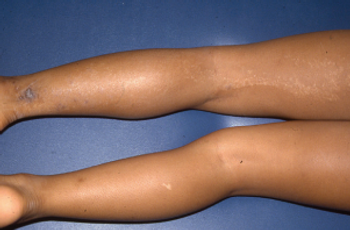
What's the diagnosis of this linear eruption on an adolescent girl's leg?

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
Pediatric cancer survivors can be vulnerable to cardiovascular disease in the short- or long-term with increased recognition of cardiotoxic cancer treatments, as 5-year survival rates for children are greater than 85%.

With 1 in 7 adolescents worldwide diagnosed with mental health disorders, emerging research suggests that diet is a powerful yet often overlooked therapeutic tool.