
The new risdiplam tablet is suitable for those aged 2 years and older who weigh more than 44 lbs, according to Genentech.

The new risdiplam tablet is suitable for those aged 2 years and older who weigh more than 44 lbs, according to Genentech.
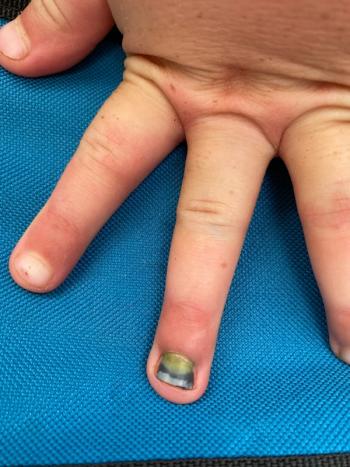
His mother reported that the nail had developed a green discoloration and had begun to detach approximately 1 week prior to the visit.

Efficacy for the kinase inhibitor was based on the ReNue (NCT03962543 ) trial that featured 58 adults and 56 pediatric patients.
A review of current guidelines in treatment and thromboprophylaxis.
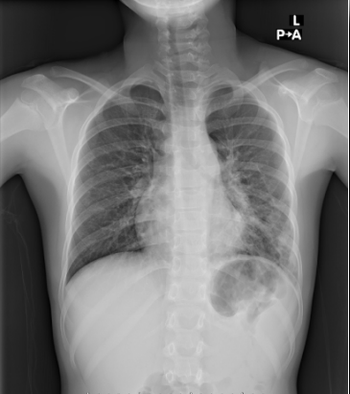
M pneumoniae infection is not a reportable disease, and testing is often not performed in the outpatient setting, limiting estimates of clinical prevalence and delaying recognition of community epidemics.

"Less pain and no need for sedation are major benefits," said Jon Matthew Farber, MD, in this edition of Journal Club.

In this discussion, leading experts weigh in on the importance of scientific integrity, proactive advocacy, and the crucial role of vaccinations in pediatric health.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.

The investigational biologic targets the interleukin-2 receptor complex in the body in order to stimulate proliferation of inhibitory immune cells.

Shriners Children’s Boston warns patients and their parents about frostbite risks, offering tips on prevention, signs, and treatment to keep kids safe in extreme cold.

Cyclo Therapeutics has enrolled 10 patients in a single-arm sub-study treating newborns to 3 years of age, evaluating the Trappsol Cyclo in the youngest subsets.

A look into nutrition's role in acne care, highlighted by Colleen Sloan, PA-C, RDN.

Allergic sensitization was more prominent in severe phenotypes but did not explain seasonal differences.

The FDA is warning diabetes patients that information from CGMs, insulin pumps, and automated dosing systems could fail to be delivered if smartphone settings are not properly configured.

The designations follow the FDA acceptance of IND application for ABO-101 to treat PH1, with a phase 1/2 study planned in the first half of 2025.

A recap of the FDA submissions and regulatory decisions in pediatrics from January 2025.

"I’m all for reducing opioids, especially in neonates, for whom we are unsure of long-term adverse effects," stated Jon Matthew Farber, MD.
The primary and all key secondary endpoints were met compared to cream vehicle in the phase 3 DELTA TEEN trial.

Application submission is based on positive phase 3 data that demonstrated improvement in patients with type 2 and 3 SMA receiving current standard of care.

As a biosimilar to Actemra, tocilizumab-anoh in both IV and SC formulation is approved to treat rheumatoid arthritis, pJIA, sJIA, COVID-19, and giant cell arteritis.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from the week, all in one place.
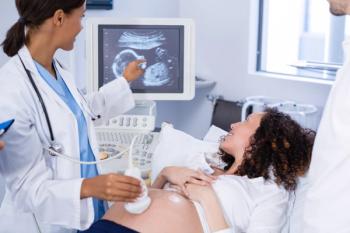
AI-assisted software improves clinicians' detection of congenital heart defects in prenatal ultrasounds, enhancing accuracy, confidence, and speed, according to a study presented at SMFM's Annual Pregnancy Meeting.

The past year saw a number of approvals adding to the toolbox for atopic dermatitis, including new formulations that work in new mechanisms.

According to a new study, racial and ethnic disparities were evident when counseling on nutrition, lifestyle, and weight among children with high blood pressure measurements.

Tina Tan, MD, FAAP, FIDSA, FPIDS, discusses the severity of the current respiratory virus season and stresses the importance of vaccinations to protect against influenza, COVID-19, RSV, and more.

"My takeaway is that if medicine is needed, stimulants will be my first choice in this age group, regardless of whether the ADHD coexists with ASD."

Can you diagnose this patient?

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the January/February, 2025, issue of Contemporary Pediatrics.

Nurse practitioner Donna Hallas, PhD, PPCNP-BC, CPNP, PMHS, FAANP, FAAN underscores how critical simple, but factual, conversations can be with parents when it comes to pediatric care.