Respiratory
Latest News
Latest Videos

CME Content
More News

Robert Frenck, MD, explained why 2024 was a big step in the right direction for RSV prevention, and emphasized the importance of vaccination against preventable diseases.

The federal agency has set a target action date of June 10, 2025 for potential approval.
The halt follows a severe respiratory disease safety signal observed in a July 2024 phase 1 trial of Moderna's mRNA-1345 and mRNA-1365 vaccine candidates.
Zelicapavir demonstrated a favorable safety profile and was well-tolerated. The N-protein inhibitor has been granted Fast Track Designation by the FDA.

Results demonstrated that those with earlier asthma onset had slower development of episodic memory.

In our latest roundtable series, we cover different therapies around COVID-19 treatment and prevention.

Get caught up with Contemporary Pediatrics! This list helps you navigate our top stories from last week, all in one place.
A further understanding of the role SARS-CoV-2 plays in T2D incidence can add an important component to benefit and risk considerations to prevent COVID-19.
The primary endpoint of the study was survival free of BPD at 36 weeks' postmenstrual age.
"The novel RSV prevention strategies may reduce ICU morbidity and mortality for children," the study authors concluded.

Octavio Ramilo, MD, highlights key developments in the effort to prevent RSV disease in recent years, highlights vaccines and monoclonal antibodies.

Octavio Ramilo, MD, joins us to discuss new clesrovimab phase 2b/3 study data presented at IDWeek 2024 in Los Angeles, California.

There were no treatment or RSV-related deaths during the study.

Contemporary Pediatrics' editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, talks RSV immunization and the availability of prevention tools.
Infants were 22% less likely to develop asthma in early childhood if there were only fed breast milk during birth hospitalization, per a study presented at the 2024 AAP National Conference & Exhibition.

The landmark indication is supported by a couple of pivotal trials assessing dupilumab for patients with chronic rhinosinusitis with nasal polyps, as well as a pediatric severe asthma trial assessing the biologic.

"This is another example of how people trying to find a useful therapeutic role for magnesium usually come up short," stated Jon Matthew Farber, MD.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the August 2024 issue of Contemporary Pediatrics.

Healthcare providers should maintain heightened suspicion of parvovirus B19 in individuals presenting with symptoms, particularly those at high risk.

An interactive text message program boosted vaping cessation rates by 35% among adolescents compared to a control group, a JAMA study found.
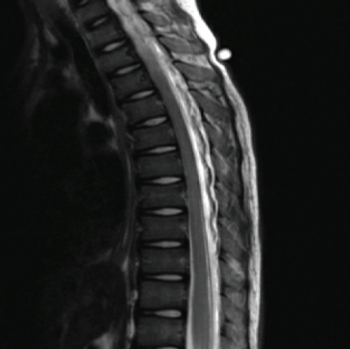
An 11-year-old boy with a history of asthma and allergic rhinitis presented to the emergency department (ED) with worsening fatigue, minimal responsivity to external stimuli, and diffuse muscle weakness for 2 months.
A pair of medical directors involved in the transplant clinical space provide commentary on heart and lung transplants in the pediatric population.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the August 2024 issue of Contemporary Pediatrics.

These data highlighted new findings on the experiences of children aged 6 to 11 with uncontrolled type 2 asthma treated with dupilumab.

These data highlighted the association of moderate air pollution exposure during pregnancy, infancy, and early childhood on pulmonary function at school age.