Respiratory
Latest News
Latest Videos
CME Content
More News

Unexpected seasonal RSV following COVID-19 highlighted the need for ongoing research to better understand transmission and ongoing impact from the pandemic.

Investigators developed an algorithm indicating that only 7.7% of patients with BHS require an ECG at BHS diagnosis, a much lower proportion than the 45.1% of those who had undergone the test in the study sample.

The 2 groups did not differ in how likely they were to fill at least 1 prescription of any antibiotic while enrolled in kindergarten.
Management of ICEP with monoclonal antibodies against IL-5 and IgE (omalizumab) could be promising according to study authors, though extent of evidence is lacking.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the June 2024 issue of Contemporary Pediatrics.

Analysis demonstrated a significant correlation between elevated levels of serum circulating magnesium and serum vitamin D with increased risk of childhood asthma.

The federal agency has accepted the sBLA for Priority Review designation, with a target action date of September 15, 2024.

The program allows participants to reserve doses and be eligible for priority shipping, among other benefits.

Editor-in-chief Tina Tan, MD, FAAP, FIDSA, FPIDS, highlights the April 2024 issue of Contemporary Pediatrics.
The new data, published in The Lancet, showed that nirsevimab substantially reduced RSV hospitalizations.
Cohort analysis shows the rate of RSV hospitalizations among children up to 5 years old skyrocketed in 2021—and then nearly doubled again in 2022—compared to pre-pandemic rates.

The monoclonal antibody was approved by the FDA on July 17, 2023 and quickly saw high demand at the onset of the RSV season.

Juanita Mora, MD, breaks down the FDA approval of benralizumab as an add-on maintenance therapy among patients with severe asthma aged 6 to 11 years.

The FDA approval of benralizumab for patients ages 6 to 11 with asthma follows the conclusions of the phase 3 TATE study.

"Our findings provide evidence for a direct association between maternal smoking during pregnancy and wheeze occurrence," wrote the authors.

Lauren Flagg, DNP, APRN, CPNP-AC, discusses severe refractory status asthmaticus based on a session presented at the NAPNAP National Conference.

The pediatric approval to treat CABP is 1 of 3 indications approved by the FDA.
Insights between the pandemics, with a highlight on the dose-response relationship, "could be valuable in preparing health care systems for future pandemics," concluded the investigators.
The investigators concluded that age-related risk differences "highlight the necessity for tailored strategies, improving understanding of and treatment development for RVIs."

Traci Gonzales, MSN, APRN, CPNP-PC, explains her session presented at the 2024 NAPNAP National Conference, highlighting the ability to diagnose and manage uncommon causes of chronic respiratory symptoms.

Investigators observe determinable and likely harmful levels of metabolite on children exposed to their parents' secondhand e-cigarette vapors.

Though a small number of infants received nirsevimab in the analysis, results support existing nirsevimab recommendations to prevent serious RSV disease in infants.

Guidance now aligns with isolation recommendations for influenza and other respiratory illnesses.
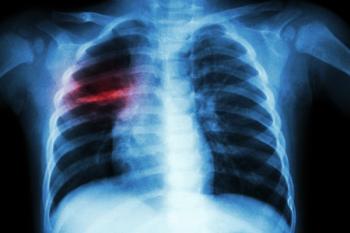
Procalcitonin can be useful for point-of-care testing in patients with influenza-like illness and a prolonged fever, guiding the indication for a chest radiograph while helping to avoid radiation exposure.

The twice-daily oral corticosteroid is indicated for 12 weeks of treatment.