Food Allergies
Latest News
Latest Videos

CME Content
More News

The newly-approved indication is for children aged 4 years and older who weigh 33 < 66 lbs, expanding on the August 9, 2024 approval in children at least 66 lbs.

Palforzia [Peanut (Arachis hypogaea) Allergen Powder-dnfp] has now launched in the US for children aged 1 through 3 years with a confirmed diagnosis of peanut allergy.

A recap of the FDA submissions and regulatory decisions in pediatrics from January 2025.

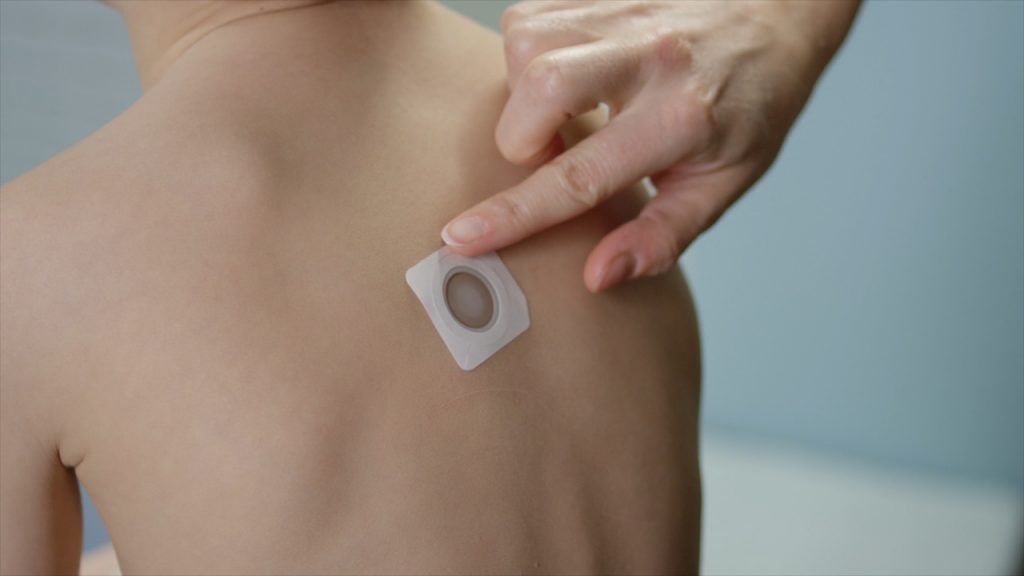
In an open-label extension of the EPITOPE phase 3 trial, the Viaskin Peanut patch had continued improvement in toddlers aged 1-3 years through 36 months.

As the authors emphasized, “Expansion of these services will be essential to meet the needs of patients experiencing food allergy-related anxiety."

Availability for the type 1 allergic reactions treatment approved by the FDA in August is expected later this month.

The approval is indicated for adult and pediatric patients who weight at least 66 pounds (30 kilograms).

Get caught up with our journal! Review some of the top stories from the Contemporary Pediatrics website over the last week, and catch up on anything you may have missed.

Peanut consumption starting in infancy resulted in lasting tolerance into adolescence.

Viaskin milk patch shows promise in cow’s milk allergy, particularly in children aged 2-11 with 300 μg dose, though the other doses examined failed to prove significant benefit.

Study authors of a paper published in The New England Journal of Medicine highlighting omalizumab for multiple common food allergies provided their comments regarding positive phase 3 data.

A poster presentation at AAAAI from the phase 3 PEOPLE Study revealed DBV712 treatment in children with peanut allergy has a favorable safety and tolerability profile.

Omalizumab was approved by the FDA on February 16, 2024 as the first and only FDA-approved medicine to reduce allergic reactions in patients with 1 or more food allergies.

Thomas Casale, MD, discusses phase 3 data presented at the 2024 AAAAI Meeting in Washington DC, highlighting neffy's efficacy in pediatric patients at risk for anaphylaxis.

In a recent phase 3 trial, omalizumab (Xolair; Genentech) led to children and adolescents being able to receive higher doses of common foods without allergic reactions.

Desensitization was observed in children aged 1 to 4 years using peanut sublingual immunotherapy (SLIT) compared to placebo, demonstrating a significantly greater median cumulative tolerated dose and higher likelihood of demonstrating remission.

This expanded upon the limited data available on risks of precautionary allergen label food introduction for those with allergies, the results of which suggest new possibilities for future allergy management.

The authors noted that there is evidence that recommending early introduction of peanut without prior testing is safe and effective in increasing early peanut introduction to infants, even those at high risk.

New data on oral immunotherapy to food allergens shows promise for the treatment, though more information with standard interventions and regimens is needed to gain certainty on the efficacy and safety.

A new study investigates taking this treatment a step further, evaluating the effect of epicutaneous immunotherapy in toddlers with known peanut allergies.

Knowing which packaged foods contain various seeds has largely been a guessing game, but a new law enacted in January 2023 changes that.

New data expands upon the growing perception that allergenic food introduction early in a child’s life may lessen the possibility of allergy developments.

Data from AAAAI 2023 suggests that gut microbial composition, function, and metabolic activity are implicated in the efficacy of peanut oral immunotherapy in children with peanut allergy.

Recent data, presented at the AAAAI annual meeting, showed that oral sesame desensitization through crushed seeds and tahini was efficacious and safe for pediatric patients.

In a recent study, the use of text messages alongside financial incentives increased epinephrine auto-injector carrying among adolescents.